PRAXBIND FAQs

PRAXBIND is a humanized monoclonal antibody fragment (Fab) indicated in patients treated with PRADAXA when reversal of the anticoagulant effects of dabigatran is needed:
- For emergency surgery/urgent procedures
- In life-threatening or uncontrolled bleeding
For intravenous use only.
The recommended dose of PRAXBIND is 5 g, provided as two separate vials, each containing 2.5 g/50 mL of PRAXBIND.
One recommended dose for all PRADAXA patients.
For intravenous use only.
Recommended Dosage:
Ready to use immediately.
- The recommended dose of PRAXBIND is 5 g, provided as two separate 50 mL vials, each containing 2.5 g idarucizumab. Both vials are packaged together in one carton.
- There are limited data to support administration of an additional 5 g of PRAXBIND
- No reconstitution needed
Preparation:
- Remove both vials (each containing 2.5 g/50 mL idarucizumab) from carton.
- Ensure aseptic handling when preparing and administering the infusion
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Once solution has been removed from the vial, administration should begin promptly

|
Flexible administration for immediate reversal:
- Do not mix with other medicinal products
- A pre-existing intravenous line may be used for administration of PRAXBIND. The line must be flushed with sterile 0.9% Sodium Chloride Injection, USP solution prior to infusion. No other infusion should be administered in parallel via the same intravenous access
OPTION 1: INFUSIONHang vials and administer as two consecutive infusions1 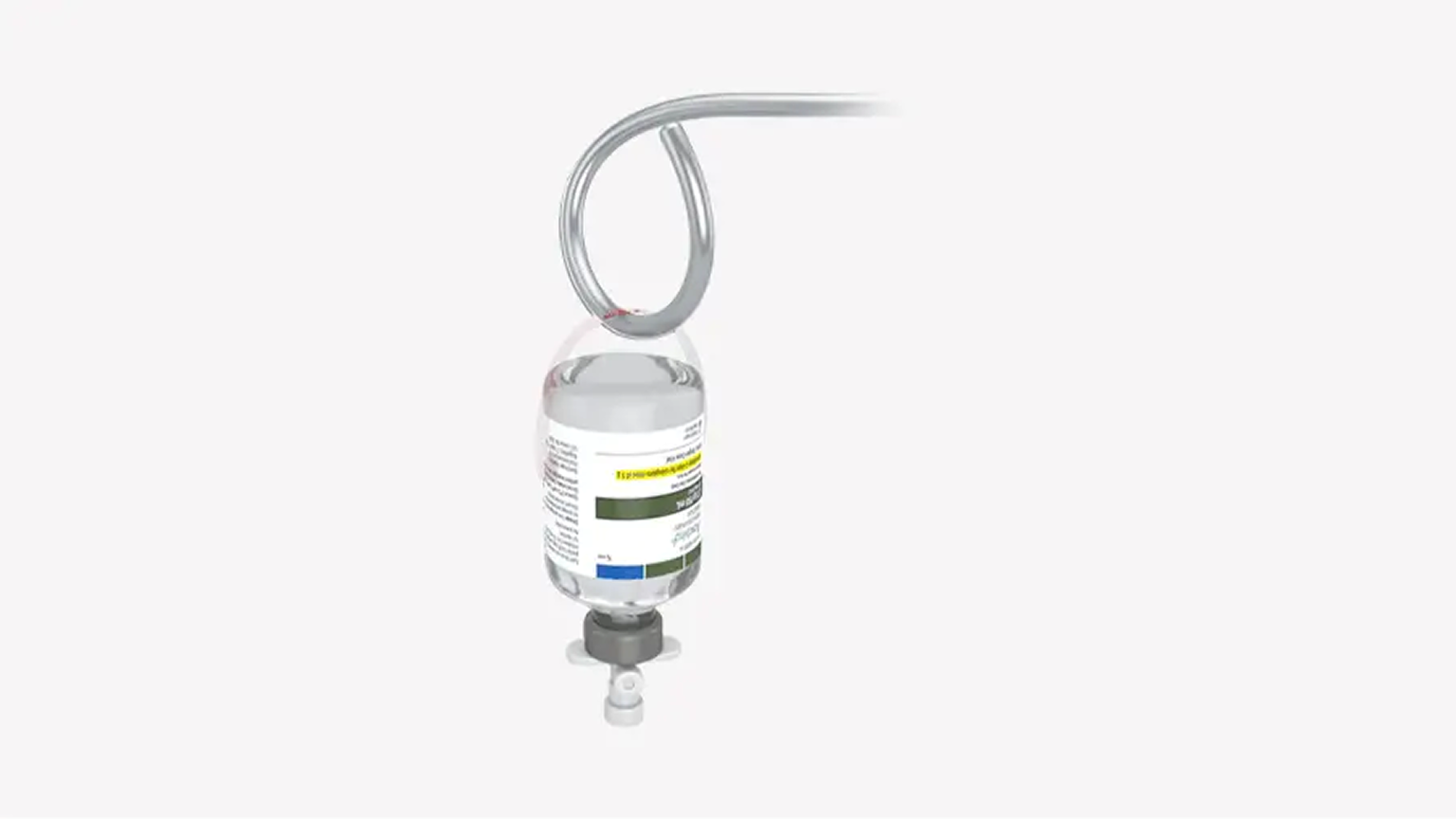
| OPTION 2: BOLUS INJECTIONInject both vials consecutively via syringe1 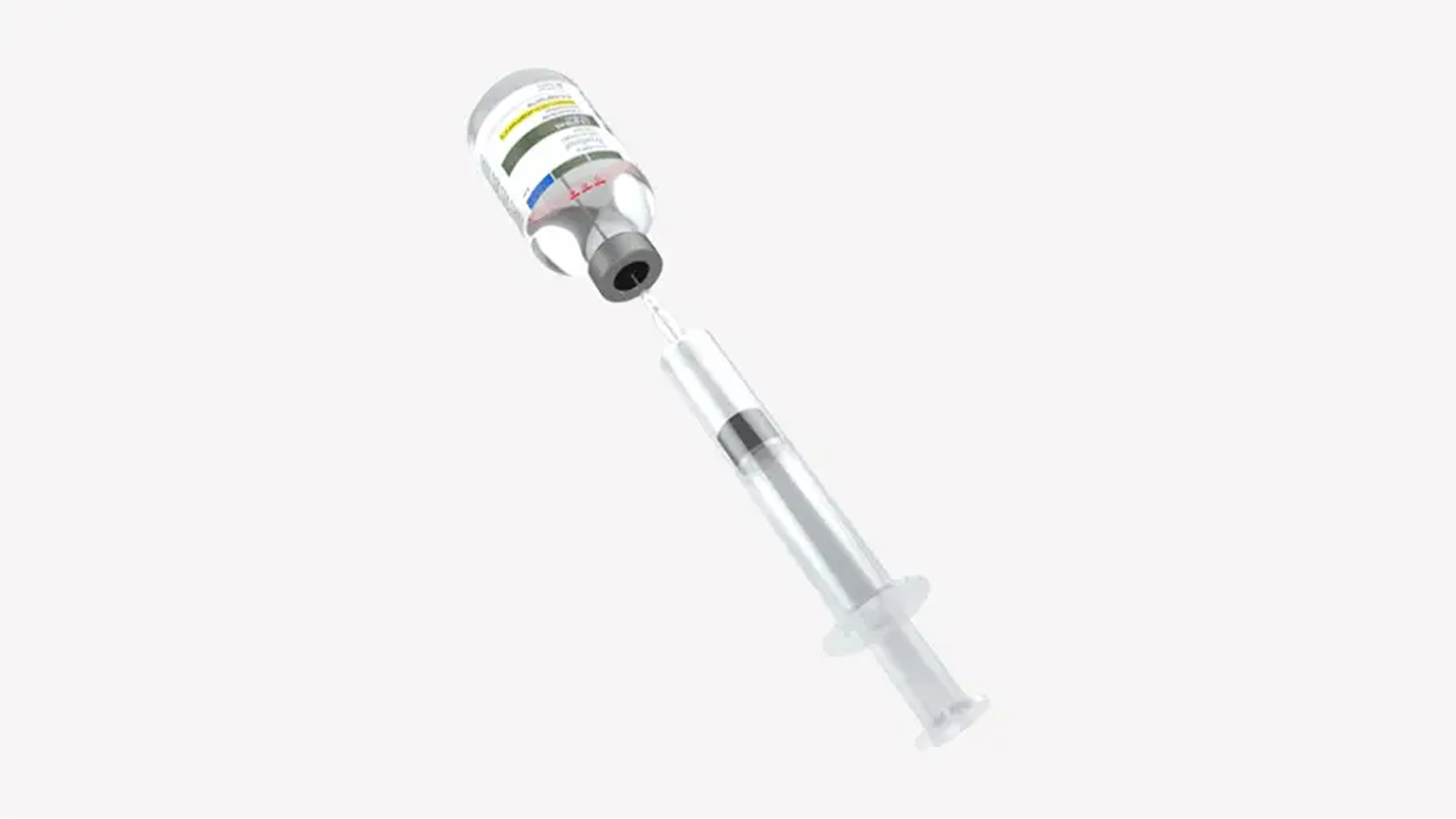
|
Watch Prep & Administration Video
PRADAXA can be re-initiated after 24 hours following PRAXBIND administration.
PRAXBIND is a humanized monoclonal antibody fragment (Fab) that specifically binds to dabigatran and its acyl glucuronide metabolites and reverses their anticoagulant effect immediately after administration.
Watch How PRAXBIND Works
The most frequently reported adverse reactions in ≥5% of patients were constipation (7%) and nausea (5%).
Thromboembolic Risk
Inform patients that reversing dabigatran therapy exposes them to the thromboembolic risk of their underlying disease. To reduce this risk, resumption of anticoagulant therapy should be considered as soon as the patient is sufficiently stable.
Recurrence of Bleeding
Inform patients to get immediate medical attention for any signs or symptoms of bleeding.
Hypersensitivity Reactions
Inform patients of signs and symptoms of allergic hypersensitivity reactions such as anaphylactoid reactions that may be experienced during or after injection of PRAXBIND.
Risk of Serious Adverse Reactions in Patients with Hereditary Fructose Intolerance due to Sorbitol Excipient
Inform patients with hereditary fructose intolerance (HFI) that PRAXBIND contains sorbitol. Parenteral administration of sorbitol in patients who have HFI has been associated with reports of hypoglycemia, hypophosphatemia, metabolic acidosis, increase in uric acid, acute liver failure with breakdown of excretory and synthetic function, and death and may occur during or after injection of PRAXBIND.
Elimination
Renal clearance: 47.0 mL/minute (gCV 18.4%)
Initial half-life: 47 minutes (gCV 11.4%)
<1% recovered in urine after 24 hours; 32.1% after 6 hours
- Remainder of dose assumed to be eliminated via protein catabolism
Specific Populations
- Age, sex, race (Caucasian vs Asian) and body weight had no clinically important effect on exposure*
Drug Interactions
In vitro data suggest that reversal of PRADAXA is not affected by:
- Coagulation factor concentrates†
In animal studies, it was suggested that neutralization of anticoagulant activity is not influenced by 50% hemodilution with routinely used volume replacement strategies‡
For complete information on the pharmacokinetics of PRAXBIND, please see the Prescribing Information.
-
*
Based on population pharmacokinetic analyses which included data from 220 volunteers and 486 patients.
-
†
Based on in vitro assessment of 3‑ or 4‑factor prothrombin complex concentrates (PCCs), activated PCC, or recombinant Factor VIIa.
-
‡
Based on assessment of crystalloids, colloids, and retransfusion of washed red blood cells.
No dose adjustment is required in renally‑impaired patients.
- In a study of subjects with renal impairment (mild, n=12; moderate, n=6)*, the total clearance was reduced vs healthy subjects
- This led to an increase in PRAXBIND’s area under the curve (mild 43.5%; moderate 83.5%)
-
*
Mild renal impairment: creatinine clearance ≥60 to <90 mL/min by Cockcroft-Gault equation. Moderate renal impairment: ≥30 to <60 mL/min.
- Store PRAXBIND vials in the refrigerator at 2°C to 8°C (36°F to 46°F). Do not freeze. Do not shake
- Prior to use, the unopened vial may be kept at room temperature 25°C (77°F) for up to 48 hours, if it’s kept in its original packaging. This packaging protects it from light. When exposed to light, it may be kept for 6 hours
Please click here for the latest information on how to order PRAXBIND.
The Boehringer Ingelheim Pharmaceuticals, Inc. Return Goods Policy for PRAXBIND applies to products purchased from an Authorized Distributor of Record (ADR) that is duly licensed as a provider to dispense BIPI product. Please contact BI Customer Service at (800) 243‑0127 for additional details.

